Right now, COVID-19 vaccines are being rolled out at pace across the UK. People from priority groups are being vaccinated every day and the aim to vaccinate 15 million of the most vulnerable people by mid-February has now been met.
In this blog we answer some of the most common questions about the COVID-19 vaccines.
Please note we cannot answer any questions that relate to individual health concerns.
What role does a vaccine play in ending the pandemic?
Effective vaccines are a vital part of ending the COVID-19 pandemic. Through vaccination, we can stop those most at-risk from getting the virus, meaning a reduction in hospitalisations and fewer deaths. However, a vaccine is not a ‘silver bullet’ and won’t stop the pandemic immediately.
It will take time and a continued combination of all the things we know help reduce spread, such as social distancing and washing hands, a vaccine and a deeper understanding of the virus that only comes with time. Better treatments will help reduce deaths in hospitals.
How do vaccines work?
Vaccines contain either a weakened or dead version of the virus, or a part of the virus, which cannot harm the recipient.
When we receive a vaccine, it stimulates our immune system to produce antibodies like it would if we were infected with the actual` virus. These antibodies remain in our body so if we are exposed to the virus in future, we can quickly fight off the disease before we become ill.
How do we make sure vaccines are safe?
Any vaccine approved for use in the UK must go through a robust and rigorous testing process to make sure that it meets extremely high standards of safety, quality and efficacy.
Public safety always comes first and vaccines are only made available to the public when they have met strict criteria.
Vaccines, like all medicines, are highly regulated and there are checks carried out at every stage of development to ensure safety is not compromised at any point.
Once a vaccine has been developed, it is given to a small group of volunteers in a clinical trial to assess initial safety. Next, it is given to a bigger group of people, usually hundreds, to learn more about its safety and to see if an immune response is triggered. Then the trial is expanded to thousands of people and the number of people who get the disease in those vaccinated is compared to a group who did not receive the vaccine, so we can report on its safety and efficacy.
The Pfizer/BioNtech vaccine was given to 43,500 people during its clinical trial and no major side effects or safety concerns have been reported.
Who is getting vaccinated currently?
The Joint Committee on Vaccination and Immunisation (JCVI) advised that the vaccine should first be given to those living and working in care homes, followed by people over the age of 80 as well as health and social care workers. The vaccine has now been offered to all older residents of every eligible care home in England.
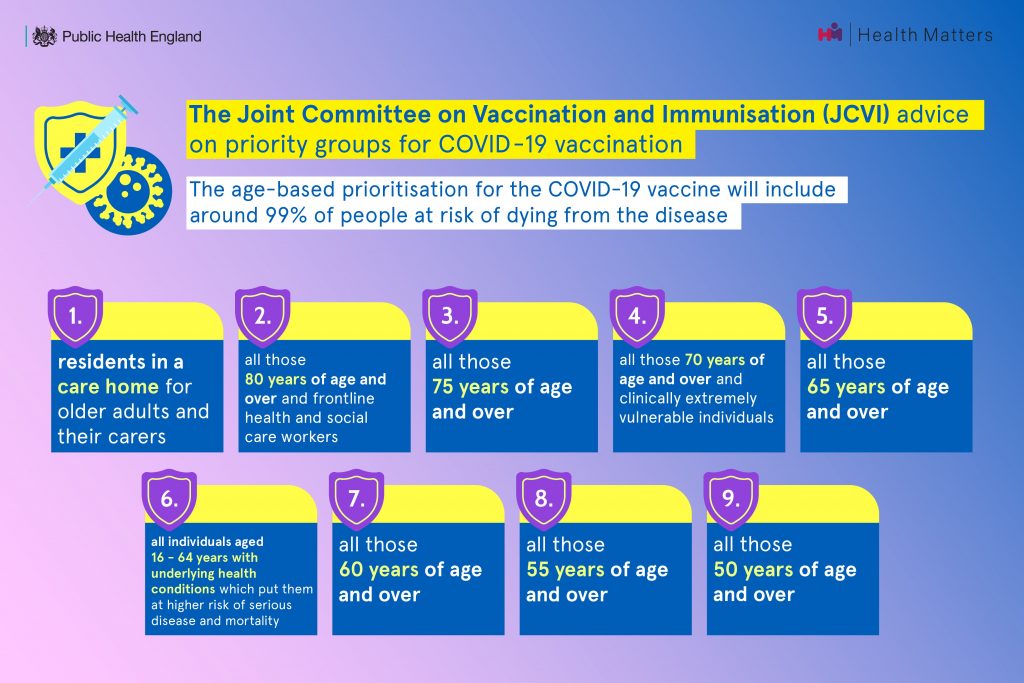
The next target is to offer vaccines to 15 million people - those aged 70 and over, healthcare workers and people required to shield - by mid-February and millions more people aged 50 and over and other priority groups by spring.
You can read more about the role of the JCVI in our explainer blog.
What about COVID-19 variants?
We are continuing efforts to understand the effect of the variants on vaccine efficacy and there is currently no evidence to suggest that vaccines will be ineffective.
We know that the vaccines currently in use are likely to have at least 50% protection against the variant first identified in South Africa, which is very encouraging. This is equivalent to flu vaccination.
We will learn more about this as the population is studied in South Africa throughout their vaccination programme.
There are a number of studies taking place at the moment including an AstraZeneca trial taking place in South Africa and we will continue to monitor the situation.
Why are we now leaving up to 12 weeks between doses of the vaccine?
Both the Oxford/AstraZeneca vaccine and Pfizer/BioNTech vaccine provide high levels of efficacy after the first dose. By giving as many people as possible the first dose of the vaccine, we are giving a greater number of people significant protection from the virus at a greater pace. This protects those who are most vulnerable and likely to suffer the worst effects of COVID-19. Simply put, every time we vaccinate someone for a second time, we are not vaccinating someone for the first time.
Why is it important to keep following the rules once you have been vaccinated?
The information we have so far on the vaccines in use are that they are highly effective, however they are not 100% effective, so there is still a chance you get infected with COVID-19, but it’s highly likely to be much less severe.
We don’t yet know if the vaccines stop you from passing the virus onto other, so while they will offer significant protection to the individual, you could still pass on COVID-19 to someone who has not been vaccinated. It is therefore important that even if you are vaccinated, you continue to follow the national guidelines to keep others safe and that if you are asked to or someone in your household has symptoms or tests positive, you still self-isolate.
Why is mixing vaccines not advised?
We do not currently recommend mixing the COVID-19 vaccines, i.e. between a first and second dose.
Patients taking part in a new clinical study will receive different COVID-19 vaccines for their first or second dose. Backed by £7 million of government funding, the study will be the first in the world to determine the effects of using different vaccines for the first and second dose - for example, using AstraZeneca’s vaccine for the first dose, followed by Pfizer/BioNTech’s vaccine for the second. Initial findings are expected to be released in the summer and the JCVI will await the results with interest.
In the meantime, we do not recommend mixing the COVID-19 vaccines.
There may be extremely rare occasions where the same vaccine is not available or it is not known which vaccine a patient received on their first dose. Every effort will be made to ensure the patient gets the same vaccine, but JCVI advice is that it is better to give a second dose of a different vaccine than to not give one at all.
What is the guidance on the Pfizer/BioNTech vaccine to people who have previously suffered allergic reactions?
Any person with a history of anaphylaxis (a serious allergic reaction) to a vaccine, medicine or food should not receive the Pfizer/BioNTech vaccine. A second dose should not be given to anyone who has experienced anaphylaxis following administration of the first dose of this vaccine.
Anaphylaxis is a known, although very rare, side effect with any vaccine. Most people will not get anaphylaxis and the benefits of protecting people against COVID-19 outweigh the risks.
Read the full guidance on the use of the Pfizer/BioNTech vaccine.
Do the current vaccines contain pork products?
No, neither the Pfizer/BioNTech or Oxford/AstraZeneca vaccines contain pork products, so they should be suitable to people of various faiths.
